
Nitrogen is the most common pure element on earth, a constituent gas of our atmosphere making up 78% of the air we breathe. Nitrogen is also the most widely used gas in industry because of its properties, colorless, tasteless, odorless, dry and non-reactive, an inert gas.
Nitrogen has 4 primary uses in industry.
- Prevent oxidation of materials
- Prevent bacteriological growth
- Reduce or remove the risk of fire and explosion
- Low dewpoint (As low as -94֯ F, -70֯ C)
The purity of nitrogen gas with respect to residual oxygen significantly affects not only the quality of the product but the cost to generate nitrogen onsite. The importance of nitrogen gas purity is the topic of this paper.
What is Nitrogen Gas purity and how is it measured:
Typically, purity is associated with cleanliness. When considering the purity of nitrogen, purity refers to residual oxygen content in the nitrogen stream. Atmospheric air is composed of various components, 78% Nitrogen (N2 ) and 21% Oxygen (O2 ) and 1% other gases. Because nitrogen and oxygen exist in high concentrations nitrogen purity only considers residual oxygen content
The majority of indutrial nitrgoen applications require 95% – 99.999% purity
Purity is typically expressed as a percentage, 95% pure nitrogen has 5% residual oxygen in the stream. 99.999% purity has 0.001% residual oxygen in the stream.
For purities above 99.9% it is more common to express purity in terms of parts per million (ppm) of residual oxygen in the N2 gas stream.

For example, the O₂ content of a 99.99% pure nitrogen gas stream will be specified as 100 ppm. Similarly, a 99.999% purity nitrogen gas stream will have an O₂ content of 10 ppm. As the purity of nitrogen increases, it is a clearer measurement to state its purity in terms of the remaining O₂ content. There is also a convention in industrial gas use to specify nitrogen purity in terms of “9s”. For example, 99.9% nitrogen purity is commonly referred to as a purity of 3 “9’s”, a gas stream of 99.999% N₂ purity containing 10 ppm O₂ would be referred to as a gas purity of 5 “9’s”
How is Nitrgoen Gas Produced On Site:
The atmosphere contains 78% nitrogen it is advantageous and practical for nitrogen gas users to invest in cost effective, safer on-site, on-demand and instantaneous nitrogen production at their own facilities, rather than relying on cryogenic N₂ delivery. Generating nitrogen oniste is 50% – 90% more cost effective. Pressure swing adsorption (PSA) or membrane production methods are generally used for the separation and enrichment of nitrogen from the atmosphere.
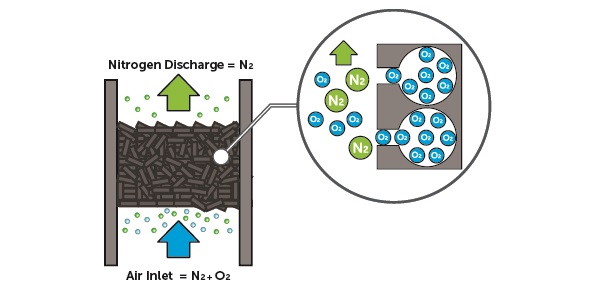
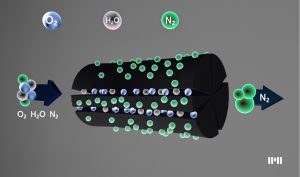 PSA nitrogen production involves a tank filled with adsorbent material, usually carbon molecular sieve (CMS). The tank is pressurized with compressed air, oxygen molecules bind to the surface of the adsorbent material, predominantly captured in pores of the CMS, the oxygen molcules are retained until the desorption process, nitrgoen molecules pass through. The pore and material diameters of the adsorbent material are important in this respect. This process results in a nitrogen-enriched gas stream with nitrogen purities ranging from 95% to 99.999%. When the adsorbent material is saturated with oxygen, desorption takes place by depressurization , oxygen molecules are released into the atmosphere. To ensure a continuous flow of nitrogen gas, PSA systems mostly use dual adsorption towers. While one tower produces nitrogen gas, the other tower desorbs O₂, preparing for the next cycle.
PSA nitrogen production involves a tank filled with adsorbent material, usually carbon molecular sieve (CMS). The tank is pressurized with compressed air, oxygen molecules bind to the surface of the adsorbent material, predominantly captured in pores of the CMS, the oxygen molcules are retained until the desorption process, nitrgoen molecules pass through. The pore and material diameters of the adsorbent material are important in this respect. This process results in a nitrogen-enriched gas stream with nitrogen purities ranging from 95% to 99.999%. When the adsorbent material is saturated with oxygen, desorption takes place by depressurization , oxygen molecules are released into the atmosphere. To ensure a continuous flow of nitrogen gas, PSA systems mostly use dual adsorption towers. While one tower produces nitrogen gas, the other tower desorbs O₂, preparing for the next cycle.
Membrane technology is the process of passing compressed air through a membrane perforated with holes with microscopic pores. These pores allow smaller O₂ molecules and water vapor to pass through the hollow fiber structure and be discharged into the atmosphere, while larger nitrgoen molecules move across the membrane, providing a continuous flow of N₂ with purities ranging from 95% to 99.9%. Most membranes are limited to 99.5% a small percentage of membtranes produced can attain 99.9%
Industrial Nitrogen Gas Applications:
The importance of oxygen content in many industrial processes strems from the fact oxdation causes adverse affects, even very low oxygen concentrations for some applications. This is due to the fact that oxygen draws electrons from other elements and causes oxidation reactions. Oxygen is responsible for many of the undesirable oxidation reactions that occur in nature and industry, such as corrosion of metallic surfaces and degradation of organic matter (food and beverage industries). Therefore, in order to control the oxidation process in industrial applications, the oxygen content is either removed or reduced to such an oxygen-nitrogen ratio that the oxygen content is not sufficient to produce harmful amounts of oxidation. The importance of nitrogen gas in many industrial applications can be illustrated with three typical examples:
Food & Beverage: Oxygen in food packages reduces shelf life. By injecting nitrogen into the packaging to displace oxygen shelf life is increased. The Food & Beverage industry worldwide has standardized on nitrogen purity of 99.5% for modified atmosphere packaging. 99.5% pure nitrogen leaves 2% or less residual oxygen in the package. Some applications such as powdered milk and wine are more sensitive to oxidation. Purities for these applications are typically 99.9%.
Electronics Soldering: The presence of even very low concentrations of oxygen during the soldering process forms metal oxides with the solder, known as “dross”, which significantly affects the soldering process. Soldering in an environment with an oxygen concentration not exceeding 10 ppm, i.e. 99.999% pure nitrogen gas, reduces dross formation, increases joint integrity and improves production quality by minimizing the need for scrap or rework.
Laser cutting: During the laser cutting process, atmospheric oxygen can oxidize the edges of the cut material, which can cause dulling or roughening of the material edges. After the cutting process, surface imperfections in the cut area can prevent coatings from being applied properly. To achieve a glossy (non-dulled) finish when cutting stainless steel, it is best to cut with high purity nitrogen, with a purity range of 99.95% to 99.999%. This means that the oxygen content should not be greater than 500 ppm to 10 ppm. The faster the cutting speed, thicker the cut higher nitrogen purity and flow is required.
For these applications, Generating nitrogen onsite using PSA technology is typically 50% – 90% less costly than tradional bulk liquid supply. PSA technology can reliably meet the purity and flow requirments for the most demanding nitrgen requirements.
Mikropor PRO Series Nitrogen Generators
Mikropor PRO Series nitrogen generators offer high efficiency to industrial plants with their compact design and fully automatic operation. By eliminating the use of manifolds, it enables the system to operate more efficiently and safely. Thanks to the touchscreen PLC display, the entire system can be easily controlled and safe production is ensured with its fast start-up feature. Thanks to the new silencer specially designed by Mikropor, it is possible to work with low noise levels during pressurization and evacuation processes. Durable piston valves ensure long life and minimum maintenance.
Mikropor MNG PRO Series Nitrogen Generators offer a low cost, high performance and energy efficient solution with nitrogen purity ranging from 95% to 99.999% according to customer requirements. It is one of the leading products in the industry with optimized air distribution and low air/nitrogen ratios in nitrogen gas production.
To choose the most suitable purity rate and capacity Mikropor MNG PRO Series Nitrogen Generator for your facility or to get more information, you can contact our expert sales teams and review our products here.
Mikropor R&D Department


